Here are some home health related updates for this week:
AMITY’S NEWS
Amity Healthcare Group Consulting Services
Amity Healthcare Group is providing continuous support to home health providers. We have been closely monitoring the events and information associated with COVID -19 vaccination mandates. If you need assistance with developing Policies & Procedures related to Mandatory COVID-19 Vaccination Requirements, please call 303-690-2749 or email at ig@amityhealthcaregroup.com
We also provide assistance with:
- Medicare Certification/Accreditation Survey preparation
- Regulatory and clinical compliance review
- QAPI implementation and analysis
- Emergency Preparedness
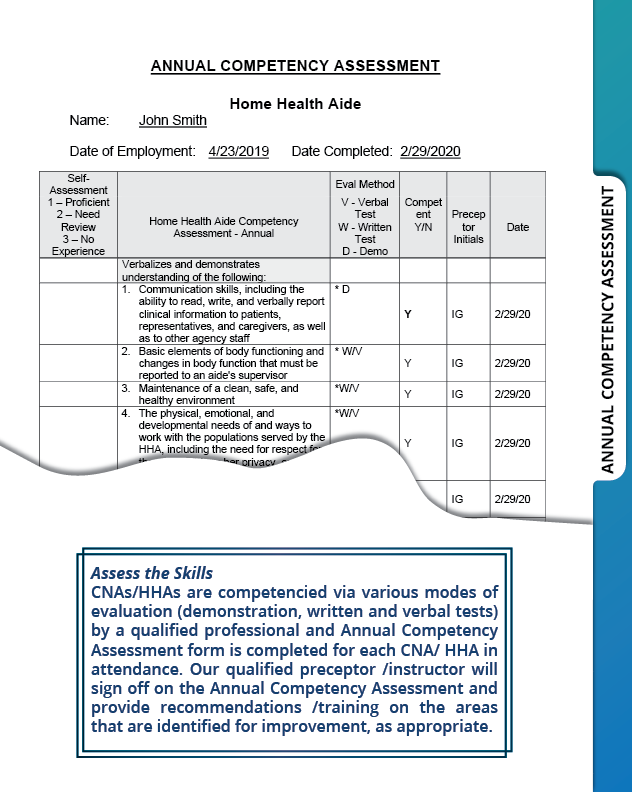
Do not forget to schedule your in-person and/or virtual competencies at https://amityhealthcaregroup.com/virtual-nursing-competency/
Join Irina Gorovaya and Amity Healthcare Group for a presentation on Practical Guide to Home Health Competency Program at Home Care and Hospice Association of Colorado Annual Conference in beautiful Keystone, Colorado October 11-12, 2021.
NATIONAL NEWS
COVID-19 Provider Funding- Phase 4
On September 10th,the Biden Administration announced that the U.S. Department of Health and Human Services (HHS), through the Health Resources and Services Administration (HRSA), will be making $25.5 billion in new funding available for health care providers affected by the COVID-19 pandemic. This funding presents a combination of $8.5 billion in American Rescue Plan (ARP) resources for providers who serve rural Medicaid, Children’s Health Insurance Program (CHIP), or Medicare patients, and an additional $17 billion for Provider Relief Fund (PRF) Phase 4 available for a wide range of providers who can document revenue loss and expenses associated with the pandemic.
Provider Relief Fund Phase 4:
- Phase 4 payments will be based on providers’ lost revenues and expenditures between July 1, 2020, and March 31, 2021.
- Phase 4 intends to reimburse smaller providers that tend to operate on thin margins and often serve vulnerable or isolated communities. In addition, Phase 4 will be inclusive of new elements specifically focused on equity, including reimbursing smaller providers for their lost revenues and COVID-19 expenses at a higher rate compared to larger providers, and bonus payments based on the amount of services providers furnish to Medicaid/CHIP and Medicare patient.
- 75% of the Phase 4 allocation will be calculated based on revenue losses and COVID-related expenses.
- Large providers will receive a minimum payment amount that is based on a percentage of their lost revenues and COVID-related expenses.
- Medium and small providers will receive a base payment plus a supplement, with small providers receiving the highest supplement, as smaller providers tend to operate on thin margins and often serve vulnerable or isolated communities.
- HHS will determine the exact amount of the base payments and supplements after analyzing data from all the applications received to ensure HHS stays within the budget and funds are distributed equitably.
- No provider will receive a Phase 4 payment that exceeds 100% of their losses and expenses.
- 25% of the Phase 4 allocation will be put towards bonus payments that are based on the amount and type of services provided to Medicaid, CHIP, and Medicare patients.
- HHS will price Medicaid and CHIP claims data at Medicare rates, with some limited exceptions for some services provided predominantly in Medicaid and CHIP.
- Providers who serve any patients living in Federal Office of Rural Health Policy-defined rural areas with Medicaid, CHIP, or Medicare coverage, and who otherwise meet the eligibility criteria, will receive a minimum payment.
American Rescue Plan:
- Providers who serve Medicaid, CHIP, and Medicare patients who live in rural communities are eligible for the ARP Rural payments.
- HHS will make payments to providers based on the amount and type of Medicare, Medicaid, and Children’s Health Insurance Program (CHIP) services provided to rural patients.
-
- HHS will price Medicaid and CHIP claims data at Medicare rates, with some limited exceptions for some services provided predominantly in Medicaid and CHIP.
- Providers who serve any patients living in Federal Office of Rural Health Policy-defined rural areas with Medicaid, CHIP, or Medicare coverage, and who otherwise meet the eligibility criteria, will receive a minimum payment.
Additional Notes:
- Provider Relief Funds recipients will be required to notify the HHS of any merger with or acquisition of another healthcare provider during their Payment Received Period. Providers who report a merger or acquisition may be more likely to be audited to confirm their funds were used for coronavirus-related costs, consistent with an overall risk-based audit strategy.
- Providers will be able to apply for both programs (PRF and ARP) in a single application. HRSA will use existing Medicaid, CHIP and Medicare claims data in calculating payments. The application portal will open on September 29, 2021
- To promote transparency in the PRF program, HHS is also releasing detailed information – PDF about the methodology utilized to calculate PRF Phase 3 payments. Providers who believe their PRF Phase 3 payment was not calculated correctly according to this methodology will have an opportunity to request a reconsideration. Further details on the PRF Phase 3 reconsideration process are forthcoming.
Reporting Requirements:
- As previously, all funds received in Phase 4 will be subject to reporting requirements with expected reporting time period of January 1 to March 31, 2023.
- Phase 1 recipients, please note that HHS announced a 60-day grace period to help providers come into compliance with their PRF Reporting requirements if they fail to meet the deadline on September 30, 2021. While the deadlines to use funds and the Reporting Time Period will not change, HHS will not initiate collection activities or similar enforcement actions for noncompliant providers during this grace period.
- While you will be out of compliance if you do not submit your report by September 30, 2021, recoupment or other enforcement actions will not be initiated during the 60-day grace period (October 1 – November 30, 2021).
- The grace period begins on October 1, 2021 and will end on November 30, 2021.
- Providers who are able are strongly encouraged to complete their report in the PRF Reporting Portal by September 30, 2021.
- Providers should return unused funds as soon as possible after submitting their report. All unused funds must be returned no later than 30 days after the end of the grace period (December 30, 2021).
This grace period only pertains to the Reporting Period 1 report submission deadline. There is no change to the Period of Availability for use of PRF payments.
For more information about eligibility requirements, the documents and information providers will need to complete their application, and the application process for PRF Phase 4 and ARP Rural payments, visit: https://www.hrsa.gov/provider-relief/future-payments.
Targeted Probe and Educate
Please remember that Targeted Probe and Educate (TPE) resumed effective September 1, 2021.
Please note that several MACs issued an updated reference to TPE.
For CGS, please go to: https://www.cgsmedicare.com/hhh/pubs/news/2021/09/cope23220.html
For Palmetto, please go to: https://www.palmettogba.com/palmetto/jmb.nsf/DID/ASNPH36180
Biden-Harris Administration to Expand Vaccination Requirements for Health Care Settings
On September 9, 2021, the Biden-Harris Administration announced that COVID-19 vaccination will be required of staff within all Medicare and Medicaid-certified facilities.
The Centers for Medicare & Medicaid Services (CMS), in collaboration with the Centers for Disease Control and Prevention (CDC), announced that emergency regulations requiring vaccinations for nursing home workers will be expanded to include hospitals, dialysis facilities, ambulatory surgical settings, and home health agencies, among others.
Furthermore, the Administration plans to include the mandate as part of the federal Medicare Conditions of Participation (CoPs).
CMS is developing an Interim Final Rule with Comment Period that will be issued in October to lay out the vaccination requirements for health care providers. Meanwhile, CMS expects Medicare and Medicaid facilities to comply with the new vaccination requirements now and health care workers in covered facilities are being urged to begin vaccination without further delay.
Additionally, the Department of Labor, through the Occupational Safety and Health Administration (OSHA), will be issuing an Emergency Temporary Standard requiring all businesses with more than 100 employees to either mandate vaccinations for all workers or require them to take weekly COVID-19 tests. Each violation of the OSHA standard could trigger a $14,000 penalty.
Amity Healthcare Group will be closely monitoring further developments in this area and update you accordingly.
Advance Beneficiary Notice- Form CMS-R-131
With the latest Paperwork Reduction Act submission, a change has been made to the ABN. In accordance with Title 18 of the Social Security Act, guidelines for Dual Eligible beneficiaries have been added to the ABN form instructions.
* Special guidance for people who are dually enrolled in both Medicare and Medicaid, also known as dually eligible individuals (has a Qualified Medicare Beneficiary (QMB) Program and/or Medicaid coverage) ONLY:
Dually Eligible beneficiaries must be instructed to check Option Box 1 on the ABN in order for a claim to be submitted for Medicare adjudication. Strike through Option Box 1 as provided below:
□ OPTION 1. I want the (D) listed above. You may ask to be paid now, but
I also want Medicare billed for an official decision on payment, which is sent to me on a Medicare Summary Notice (MSN). I understand that if Medicare doesn’t pay, I am responsible for payment, but I can appeal to Medicare by following the directions on the MSN.
These edits are required because the provider cannot bill the dual eligible beneficiary when the ABN is furnished. Providers must refrain from billing the beneficiary pending adjudication by both Medicare and Medicaid in light of federal law affecting coverage and billing of dual eligible beneficiaries.
If Medicare denies a claim where an ABN was needed in order to transfer financial liability to the beneficiary, the claim may be crossed over to Medicaid or submitted by the provider for adjudication based on State Medicaid coverage and payment policy. Medicaid will issue a Remittance Advice based on this determination.
Once the claim is adjudicated by both Medicare and Medicaid, providers may only charge the patient in the following circumstances:
• If the beneficiary has QMB coverage without full Medicaid coverage, the ABN could allow the provider to shift financial liability to the beneficiary per Medicare policy.
• If the beneficiary has full Medicaid coverage and Medicaid denies the claim (or will not pay because the provider does not participate in Medicaid), the ABN could allow the provider to shift financial liability to the beneficiary per Medicare policy, subject to any state laws that limit beneficiary liability.
Note: These instructions should only be used when the ABN is used to transfer potential financial liability to the beneficiary and not in voluntary instances.
Educational Opportunities
Home Care Vaccine Mandate Discussion with Industry Leaders and Legal Experts
NAHC Education is offering a free webinar on Monday, September 20th to discuss how COVID-19 continues to have an impact on the home care communities across the United States. This webinar is an opportunity to join NAHC and other industry leaders as well as legal experts from Polsinelli for an open conversation about vaccine mandates and the impact they could have and are already having on home health industry.
COLORADO NEWS
The COVID-19 Vaccination Mandates
On September 15, 2021, the Colorado Department of Public Health and Environment released new guidance outlining mandatory staff vaccination for all licensed healthcare facilities.
Please note the following:
- Exemptions:
Religious exemptions – The criteria for religious exemptions is determined by facility policy. Religious exemptions must be documented and retained for inspection by Health Facility investigators. A single religious exemption in the facility requires the facility to submit a waiver request of the 100% vaccination requirement.
Medical exemptions – A medical exemption signed by a physician, physician assistant, advanced practice nurse, or certified nurse midwife licensed in the State of Colorado stating that the COVID-19 vaccination for the employee, direct contractor, or support staff is medically contraindicated as described in the product labeling approved or authorized by the Food and Drug Administration (FDA). Medical exemptions must be documented and retained for inspection by Health Facility investigators. Medical exemptions do not require the facility to submit a waiver request of the 100% vaccination requirement.
- Waivers:
Waiver Requests
Facilities may seek a waiver request of the 100% vaccination requirement under the following
conditions:
| Waiver Required | No Waiver Required | ||
| The facility has more than zero (0) religious exemptions. |
100% of employees, direct contractors, and support staff are vaccinated. |
||
| The combination of vaccinated staff and staff with documented medical exemptions accounts for 100% of the facility’s employees, direct contractors, and support staff. |
Facilities may begin applying for waivers through the Health Facilities and Emergency Medical
Services Division beginning September 17, 2021. CDPHE will be providing additional instructions and application for the waiver. Waivers may not be submitted prior to that date.
- Mitigation Strategies for Unvaccinated Personnel
** Source Control Measures
** Testing Frequency
For a complete guidance, please visit: file:///C:/Users/eduar/AppData/Local/Temp/Mandatory%20Staff%20Vaccination%20Guidance.pdf
Amity Healthcare Group submitted additional questions to CDPHE and Residential Care Strike Team as related to the issued guidance and will keep you updated.
Private Duty Nursing and Pediatric Long-Term Home Health PARs
Prior authorization request (PAR) requirements were suspended for private duty nursing (PDN) and pediatric long-term home health (PLTHH) on July 1, 2020. As per communication from Gainwell Technologies on September 9, 2021, PAR submission will not resume until November 1, 2021.
Effective November 1, 2021, providers will be required to submit PARs to the new utilization management (UM) vendor Kepro via the online PAR portal, Atrezzo. A phased-in approach of at least six months has been agreed upon but a longer phased-in implementation is currently being evaluated. Providers will be responsible for submitting a percentage of their PARs throughout the month for each phase of implementation, and providers will be responsible for deciding how to organize PAR submissions. Additional guidance regarding the implementation plan and timeline will be provided via the provider bulletin, the PDN/PLTHH Project web page and the ColoradoPAR Program web page.
The Department of Health Care Policy & Financing (the Department) will:
- Issue a 60-day administrative approval prior to any denial going into effect
- Issue continuation of benefits for any member appeal as normal
- Step down process for PDN denial that reduce services by 30% or more will continue as normal
- Send weekly reports to Regional Accountable Entities (RAEs) of any denials to allow for care coordination
Note: While the UM vendor has changed, the policy for these two benefits remained unchanged. Providers are required to submit the Pediatric Assessment tool for PLTHH PARs, and the PDN Acuity Tool for PDN PARs.
Amity’s Friday emails (newsletters) will be archived on Amity’s Healthcare Group website at https://amityhealthcaregroup.com/resources/ under Weekly Newsletter section.
Please do not hesitate to reach out for any assistance or questions via email, phone or website at https://amityhealthcaregroup.com/resources/
If you wish to forward this email to your colleague or friend, please feel free to do so. If you received this message as a forward, we invite you to subscribe to our communications at https://amityhealthcaregroup.com/ (look for “Subscribe to Listserv” in the top corner). If you wish to unsubscribe from this email distribution list, please email to eg@amityhealthcaregroup.com